Study Center: New York
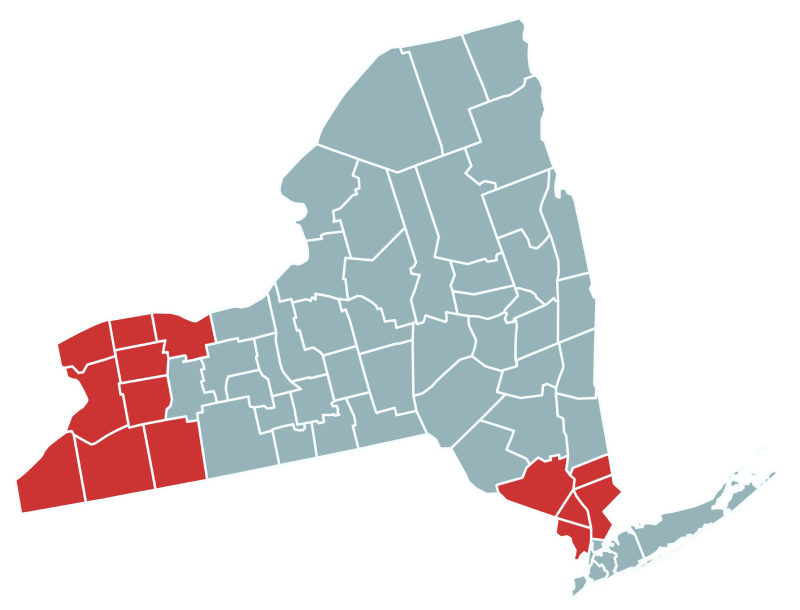
The New York State Department of Health operates the second largest statewide, population-based birth defects registry in the nation. The New York Center for Birth Defects Research and Prevention has participated in CDC-funded research on birth defects since 1997. For NBDPS and BD-STEPS, the New York Center focuses on pregnancies impacted by birth defects in counties in Southern and Western NY.
The New York Center has expertise in studying medication use in pregnancy as well as examining exposures in the workplace and the environment that might lead to birth defects. We have established collaborations with the Wadsworth Center, the research-intensive public health laboratory of the New York State Department of Health to use newborn screening blood spots for research on genetic and environmental risk factors for birth defects.
We appreciate the participation of over 3,000 New York families in the NBDPS, the largest study of birth defects in the United States.
Contact
Phone: 888-296-8192
E-mail: ny@nbdps.org
Principal Investigators

Meredith Howley, PhD, SM
Research Scientist
Meredith Howley, PhD, SM is a Research Scientist at the New York State Department of Health and has worked with the New York Center for Birth Defects Research and Prevention since 2014. In her role as PI, she oversees implementation of BD-STEPS in NY, sets the research agenda of the Center, and works to train future birth defects researchers. . Additionally, Dr. Howley is a Research Assistant Professor in the Department of Epidemiology at the University at Albany’s School of Public Health. Dr. Howley’s research focuses on maternal disease and medication use during pregnancy and their potential impacts on the risk of birth defects. Her recent work has focused on maternal chronic conditions and/or their treatments, including asthma, autoimmune diseases, and insomnia.
Sarah Fisher, PhD, MPH
Research Scientist
Sarah Fisher, PhD, MPH is a Research Scientist in the Office of the Director of the Bureau of Environmental and Occupational Epidemiology at the New York State Department of Health. As co-Principal Investigator of the NY Center, Dr. Fisher co-directs the Center’s research agenda and implementation of BD-STEPS in New York. Dr. Fisher’s research interests include understanding the effect of alcohol use on the developing fetus and studying the relationships between chronic maternal conditions, such as hypertension, and the medications used during pregnancy to treat those conditions, and risk of birth defects.

Former Principal Investigators

Marilyn L. Browne, PhD
Principal Investigator
Marilyn L. Browne, PhD served as the Principal Investigator of the New York Center for Birth Defects Research and Prevention for over 10 for years, while also serving as the Director of Birth Defects Research at the New York State Department of Health and as an Associate Professor in the Department of Epidemiology at the University at Albany’s School of Public Health. For the duration of the NBDPS, Charlotte Druschel MD, MPH was the Medical Director of the New York State Congenital Malformations Registry and the Principal Investigator of the New York Center. Erin Bell, PhD, an Associate Professor in the Department of Epidemiology at the University at Albany’s School of Public Health, also served as a co-Principal Investigator during the NBDPS.
Local Activities and Research
As part of the National Birth Defects Prevention Study, the New York Center led efforts to study medication use in pregnancy, maternal disease and infection, alcohol use, and environmental exposures and how these might impact the risk of birth defects. Specific projects have explored:
- Untreated chronic diseases, like hypertension, thyroid disease, and asthma that could pose a risk to the mother and baby. Our studies of maternal illness and medication use can help women and physicians make informed decisions about medication use during pregnancy.
- The effects of certain behaviors during pregnancy, including alcohol use and caffeine use on the risk of various birth defects.
- Occupation and environmental exposures (like air pollution and tap water) and the risk of birth defects. Since we are based in the Department of Health’s Center for Environmental Health, a recipient of an Environmental Public Health Tracking award, we have used these resources to further study environmental exposures.
Notable Research Findings
Papadopoulos EA, Howley MM, Fisher SC, Van Zutphen AR, Werler MM, Romitti PA, Browne ML. Antifungal medication use during pregnancy and the risk of selected major birth defects in the National Birth Defects Prevention Study, 1997-2011. Pharmacoepidemiol Drug Saf. 2024 Jan; 33(1): e5741. doi: 10.1002/pds.5741.
Howley MM, Werler MM, Fisher SC, Tracy M, Van Zutphen AR, Papadopoulos EA, Hansen C, Ailes EC, Reefhuis J, Wood ME, Browne ML. Maternal exposure to zolpidem and risk of specific birth defects. J Sleep Res. 2024 Feb; 33(1):e13958. doi: 10.1111/jsr.13958.
Fisher SC, Romitti PA, Tracy M, Howley MM, Jabs EW, Browne ML. Associations between maternal periconceptional alcohol consumption and risk of omphalocele among offspring, National Birth Defects Prevention Study, 1997-2011. Prev Med. 2024 Feb 9: 180:107891. doi: 10.1016/j.ypmed.2024.107891.
Williford EM, Howley MM, Fisher SC, Conway KM, Romitti PA, Reeder MR, Olshan AF, Reefhuis J, Yazdy MM, Browne ML. Maternal Caffeine Consumption and Risk of Birth Defects in the National Birth Defects Prevention Study, 1997-2011. Birth Defects Res. 2023 May 15; 115(9):921-932. doi: 10.1002/bdr2.2171.
Fisher SC, Howley MM, Tran EL, Ailes EC, Papadopoulos EA, Nembhard WN, Browne ML. Maternal Cyclobenzaprine Exposure and Risk of Birth Defects in the National Birth Defects Prevention Study (1997-2011) and Birth Defects Study to Evaluate Pregnancy Exposures (2014-2018). Pharmacoepidemiol Drug Saf. 2023 Aug; 32(8):855-862. doi: 10.1002/pds.5619.
Howley MM, Papadopoulos EA, Van Bennekom CM, Can Zutphen AR, Carmichael SL, Munsie JW, Herdt ML, Browne ML. Asthma Medication Use and Risk of Birth Defects: National Birth Defects Prevention Study, 1997–2011. J Allergy Clin Immunol Pract. 2020 Nov-Dec; 8(10): O3490-3499.e9. doi: 10.1016/j.jaip.2020.07.033.